- About us
- Research
- Students & Teaching
- Seminars & Events
- Directories
- Booking Rooms & Equipment
- עברית
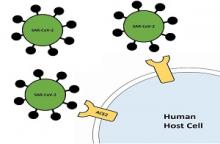
Home » Prof. Linial, Dr. Schneidman and Dr. Brielle show that the SARS-CoV-2 exerts a distinctive strategy for interacting with the ACE2 human receptor