- About us
- Research
- Students & Teaching
- Seminars & Events
- Directories
- Booking Rooms & Equipment
- עברית
Home » Dr. Eitan Lerner has developed an innovative method for rapid analysis of biomolecule structure changes
Biomolecules, the machinery of life, are very active, and change shapes (conformations) rapidly - bending, jiggling and wiggling. This complicates studying how they work, how things go wrong in diseases, and how other molecules (such as potential pharmaceuticals) affect them.
One way of observing these motions is mediated via little probes, called fluorophores, attached to the molecules. These probes behave like little cell phones, which can send messages back to scientists in the form of photons. Understanding this data can be like guessing what a person is doing based on the type of messages they are sending:
and like a person sending emails and texts, the photons can arrive at any time. This data is therefore rather messy and hard to understand.
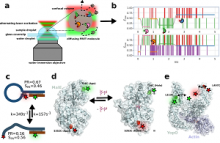
To assist this process Dr. Lerner and his post-doctoral associate Dr. Paul Harris have developed multi-parameter photon-by-photon hidden Markov modeling (mpH2MM), a novel analysis framework summarized in a recently accepted Nature Communications paper.
This framework works at the level of each photon, each message sent back by the fluorophores, the little probes attached to the molecules, and so can predict how quickly the molecule of interest changes shapes.
Drs Lerner and Harris verified their new tool worked properly and showed its potential using three key systems.
The first was a small loop of DNA whose motions were already well understood. This let them know that mpH2MM was working properly.
Then they joined forces with collaborators in Germany from the Cordes lab. Here they looked at two different proteins that both bind to specific molecules, and in the process change shapes. They found that these two proteins used very different mechanisms to bind their targets. MalE, which binds maltose, waited to see its target before changing shape, like someone who only begins a task when they see a message. The YopO, a protein from the bacterial strains that caused the bubonic plague, which hijacks host-cell signaling, was constantly bouncing back and forth between two shapes, even when its partner was absent, like someone bouncing between several tasks, but sticking with one longer when they see that it is productive.
The new method excels in two ways:
Drs. Lerner and Harris are now working on extending this technique to incorporate even more observables and applying it to different systems. The take home message is that mpH2MM is a very versatile framework, with the potential to reveal the motions of the molecules that make our bodies work.
Read the paper in Nature Communications: https://doi.org/10.1038/s41467-022-28632-x